Optimizing Best Practices for LVAD Patient Management: Perspectives of Key Opinion Leaders in Mechanical Circulatory Support
Discover What’s New: HVAD Webinar Series On-Demand
Discover KOL perspectives on best practices in VAD therapy. Watch an on-demand, comprehensive, 5-part webinar series hosted by Medtronic MCS.
Optimizing HVAD Patient Outcomes: The Texas Heart Experience
Originally broadcast July 23, 2020Brief Statement HeartWare™ HVAD™ System
Indications For Use:The HeartWare™ HVAD™ System is indicated for hemodynamic support in patients with advanced, refractory left ventricular heart failure; either as a Bridge to Cardiac Transplantation (BTT), myocardial recovery, or as Destination Therapy (DT) in patients for whom subsequent transplantation is not planned.
Contraindications: The HeartWare System is contraindicated in patients who cannot tolerate anticoagulation therapy. Warnings/Precautions: Proper usage and maintenance of the HVAD™ System is critical for the functioning of the device. Serious and life-threatening adverse events, including stroke, have been associated with use of this device. Blood pressure management may reduce the risk of stroke. Never disconnect from two power sources at the same time (batteries or power adapters) since this will stop the pump, which could lead to serious injury or death. At least one power source must be connected at all times. Always keep a spare controller and fully charged spare batteries available at all times in case of an emergency. Do not disconnect the driveline from the controller or the pump will stop. Avoid devices and conditions that may induce strong static discharges as this may cause the VAD to perform improperly or stop. Magnetic resonance imaging (MRI) could cause harm to the patient or could cause the pump to stop. The HVAD™ Pump may cause interference with automatic implantable cardioverter-defibrillators (AICDs), which may lead to inappropriate shocks, arrhythmia, and death. Chest compressions may pose a risk due to pump location and position of the outflow graft on the aorta — use clinical judgment. If chest compressions have been administered, confirm function and positioning of HVAD Pump post-CPR.
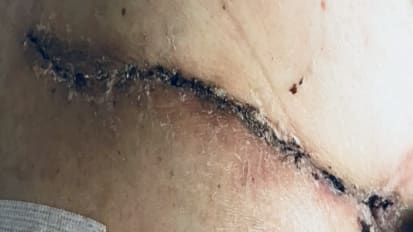
Potential Complications: Implantation of a VAD is an invasive procedure requiring general anesthesia and entry into the thoracic cavity. There are numerous known risks associated with this surgical procedure and the therapy including, but not limited to, death, stroke, neurological dysfunction, device malfunction, peripheral and devicerelated thromboembolic events, bleeding, right ventricular failure, infection, hemolysis, and sepsis. Refer to the “Instructions for Use” for detailed information regarding the implant procedure, indications, contraindications, warnings, precautions, and potential adverse events prior to using this device.
Caution: Federal law (USA) restricts these devices to sale by or on the order of a physician.
Indications, Safety and Warnings
Outside US Statement: HeartWare™ HVAD™ System
Indications for Use
The HVAD™ System is intended for use in patients at risk of death from refractory end-stage heart failure. The HVAD™ System is designed for in-hospital and out-of-hospital settings, including transportation via fixed wing aircraft or helicopter.
Contraindications
The HVAD™ System is contraindicated:
- In patients with a body surface area (BSA) less than 1.2m2
- In patients who cannot tolerate anticoagulation therapy
- During pregnancy
Warnings/Precautions
Proper usage and maintenance of the HVAD™ System is critical for the functioning of the device. Serious and life threatening adverse events, including stroke, have been associated with use of this device. Blood pressure management may reduce the risk of stroke. Never disconnect from two power sources at the same time (batteries or power adapters) since this will stop the pump, which could lead to serious injury or death. At least one power source must be connected at all times. Always keep a spare controller and fully charged spare batteries available at all times in case of an emergency. Do not disconnect the driveline from the controller or the pump will stop. Avoid devices and conditions that may induce strong static discharges as this may cause the VAD to perform improperly or stop. Magnetic resonance imaging (MRI) could cause harm to the patient or could cause the pump to stop. The HVAD™ Pump may cause interference with automatic implantable cardioverter-defibrillators (AICDs), which may lead to inappropriate shocks, arrhythmia and death. Chest compressions may pose a risk due to pump location and position of the outflow graft on the aorta -use clinical judgment. If chest compressions have been administered, confirm function and positioning of HVAD Pump post CPR. Potential Complications Implantation of a VAD is an invasive procedure requiring general anesthesia and entry into the thoracic cavity. There are numerous known risks associated with this surgical procedure and the therapy including, but not limited to, death, stroke, neurological dysfunction, device malfunction, peripheral and device-related thromboembolic events, bleeding, right ventricular failure, infection, hemolysis and sepsis. Refer to the “Instructions for Use” for detailed information regarding the implant procedure, indications, contraindications, warnings, precautions and potential adverse events prior to using this device.